Page 10 - 09 EMA
P. 10
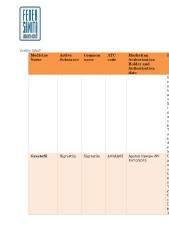
Centro Studi Active Common ATC Marketing In
Medicine code Authorisation
Name Substance name Holder and fo
Authorisation m
Grastofil date n
b
filgrastim filgrastim L03AA02 Apotex Europe BV p
18/10/2013 si
M
In
n
a
of
in
T
se
in
d
in
b
G
a
es
of
th
m
co
T
re
G
ce
In
id
1
Medicine code Authorisation
Name Substance name Holder and fo
Authorisation m
Grastofil date n
b
filgrastim filgrastim L03AA02 Apotex Europe BV p
18/10/2013 si
M
In
n
a
of
in
T
se
in
d
in
b
G
a
es
of
th
m
co
T
re
G
ce
In
id
1